BioGel XR
BioGel XR consists of a combination of a tri block copolymer and a natural polysaccharide. The system is designed to take advantage of body temperature to undergo sol-to-gel transition and can be presented, to name a few, as a liquid (viscous or dilute), a semi-solid (gel/paste), a spray, or a foam to suite the need/purpose. BioGel XR meets the biocompatibility testing requirements of ISO/USP and patents covering the formulation are published.
In-situ gelation
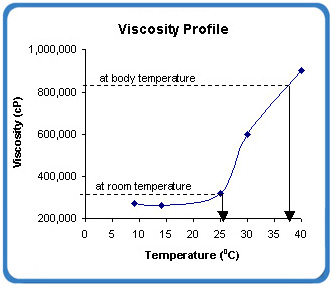
TriLogic’s proprietary in-situ gel system (BioGel XR) is a viscous liquid and undergoes solution to gel (sol-to-gel) transition using physiological condition such as body temperature as the trigger. This property allows the Company’s in-situ gel platform to (a) be easily and directly placed on or at the site of action and (b) transform to a gel following application. By the process of “reverse thermal gelation”, the viscosity of BioGel XR increases 2 – 4x of its viscosity at room temperature.
Sustained-erosion
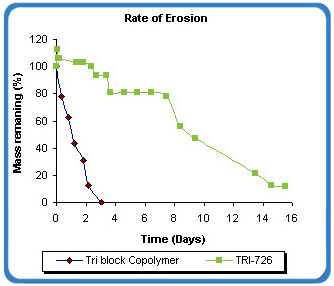
The platform has the ability to erode over a period of several hours to several days. This feature allows for the extended residence of the matrix over this period. In the example below, the erosion rate of the BioGel XR matrix is measured as an estimate of the duration of matrix residence time. The matrix erodes over 15 days under controlled condition, which represents a 5-fold increase over the tri block copolymer alone.
Bioresorption adhesion
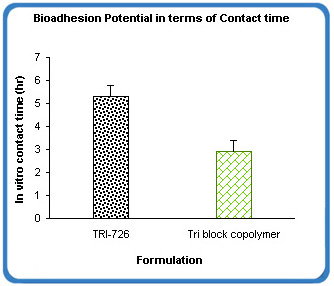
BioGel XR uses ingredients that are biocompatible and hydrophilic, making the formulation slowly dissolve in body fluids (intra and extracellular). This means that it does not require subsequent removal at the end of a treatment period. The platform exhibits excellent ability to bioadhere at site of application. BioGel XR showed excellent bioadhesion potential measured in terms of contact time which was approximately 2x that of the tri block copolymer alone.
BioGel XR consists of a combination of a tri block copolymer and a natural polysaccharide. The system is designed to take advantage of body temperature to undergo sol-to-gel transition and can be presented, to name a few, as a liquid (viscous or dilute), a semi-solid (gel/paste), a spray, or a foam to suite the need/purpose. BioGel XR meets the biocompatibility testing requirements of ISO/USP and a PCT patent application covering the formulation has been published.
BioGel XR provides the convenience of:
- Applying medications to hard-to-reach areas.
- Local action. Avoids the complications of either systemic or oral therapy such as joint pain, gastric discomfort, etc.
- Having less viscosity during initial application allowing the product to reach the various minute crevices found in tissue providing more surface coverage.
- Being biocompatible and bio-eroding. The body eliminates it through normal metabolic means.
- Being available in easy to use form makes it easier to apply as opposed to many conventional or traditional devices.